Lub roj teeb lithium-ion lossis Li-ion roj teeb (lub npe hu ua LIB) yog hom roj teeb uas siv tau.Cov roj teeb lithium-ion feem ntau yog siv rau cov khoom siv hluav taws xob thiab tsheb hluav taws xob thiab tab tom loj hlob hauv kev muaj koob npe rau kev siv tub rog thiab aerospace.Tus qauv Li-ion roj teeb tau tsim los ntawm Akira Yoshino hauv xyoo 1985, raws li kev tshawb fawb yav dhau los los ntawm John Goodenough, M. Stanley Whittingham, Rachid Yazami thiab Koichi Mizushima thaum xyoo 1970-1980s, thiab tom qab ntawd cov roj teeb Li-ion lag luam tau tsim los ntawm ib Sony thiab Asahi Kasei pab pawg coj los ntawm Yoshio Nishi hauv 1991. Hauv 2019, Nobel nqi zog hauv Chemistry tau muab rau Yoshino, Goodenough, thiab Whittingham "rau kev txhim kho cov roj teeb lithium ion".
Hauv cov roj teeb, lithium ions txav los ntawm qhov tsis zoo electrode los ntawm electrolyte mus rau qhov zoo electrode thaum tawm, thiab rov qab thaum them nyiaj.Li-ion roj teeb siv ib qho intercalated lithium compound ua cov khoom ntawm qhov zoo electrode thiab feem ntau graphite ntawm qhov tsis zoo electrode.Lub roj teeb muaj lub zog siab ntom ntom ntom, tsis muaj lub cim xeeb (lwm yam dua li LFP hlwb) thiab qis dua tus kheej.Txawm li cas los xij, lawv tuaj yeem ua rau muaj kev phom sij rau kev nyab xeeb vim lawv muaj cov hluav taws xob hluav taws xob, thiab yog tias puas lossis raug them tsis raug tuaj yeem ua rau tawg thiab hluav taws.Samsung raug yuam kom rov qab Galaxy Note 7 handsets tom qab lithium-ion hluav taws, thiab tau muaj ntau qhov xwm txheej cuam tshuam nrog roj teeb ntawm Boeing 787s.
Chemistry, kev ua tau zoo, tus nqi thiab cov yam ntxwv kev nyab xeeb sib txawv ntawm LIB hom.Handheld Electronics feem ntau yog siv cov roj teeb lithium polymer roj teeb, uas muaj cov khoom siv hluav taws xob, tab sis qhia txog kev nyab xeeb kev pheej hmoo, tshwj xeeb yog thaum puas.Lithium hlau phosphate (LiFePO4), lithium manganese oxide (LiMn2O4, Li2MnO3, los yog LMO), thiab lithium nickel manganese cobalt oxide (LiNiMnCoO2 lossis NMC) muab lub zog qis dua tab sis lub neej ntev dua thiab tsis tshua muaj hluav taws kub lossis tawg.Cov roj teeb zoo li no tau siv dav rau cov cuab yeej hluav taws xob, khoom siv kho mob, thiab lwm yam haujlwm.NMC thiab nws cov derivatives tau siv dav hauv tsheb fais fab.
Kev tshawb fawb thaj chaw rau cov roj teeb lithium-ion suav nrog kev ua neej ntev, ua kom lub zog ceev, txhim kho kev nyab xeeb, txo nqi, thiab nce kev them nqi ceev, thiab lwm yam.Kev tshawb fawb tau ua tiav nyob rau hauv thaj tsam ntawm cov tsis-flammable electrolytes raws li txoj hauv kev kom muaj kev nyab xeeb ntxiv raws li cov nplaim taws thiab tsis muaj zog ntawm cov kuab tshuaj organic siv hauv cov khoom siv electrolyte.Cov tswv yim muaj xws li aqueous lithium-ion roj teeb, ceramic khoom electrolytes, polymer electrolytes, ionic kua, thiab hnyav fluorinated systems.
Roj teeb piv rau cell
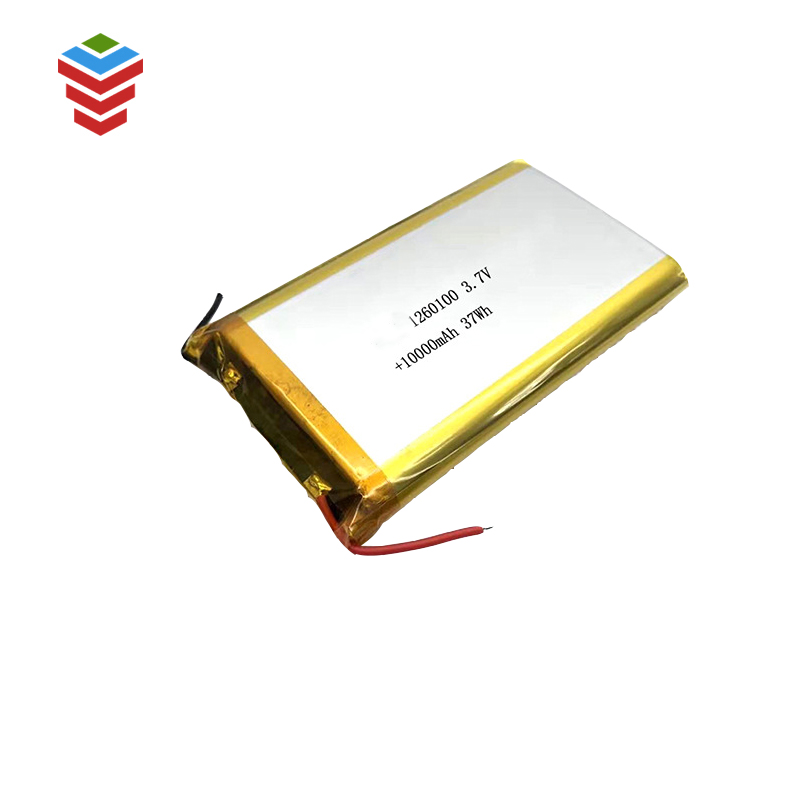

Lub xovtooj ntawm tes yog ib chav tsev electrochemical uas muaj cov electrodes, cais, thiab electrolyte.
Lub roj teeb lossis lub roj teeb pob yog ib qho kev sib sau ntawm cov hlwb lossis cov xovtooj ntawm tes, nrog cov vaj tsev nyob, kev sib txuas hluav taws xob, thiab tej zaum cov khoom siv hluav taws xob rau kev tswj thiab kev tiv thaiv.
Anode thiab cathode electrodes
Rau cov hlwb rechargeable, lub sij hawm anode (los yog tsis zoo electrode) xaiv cov electrode qhov twg oxidation tshwm sim thaum lub sij hawm tawm mus;Lwm electrode yog cathode (los yog zoo electrode).Thaum lub sij hawm lub voj voog, qhov zoo electrode dhau los ua tus anode thiab qhov tsis zoo electrode ua cathode.Rau feem ntau lithium-ion hlwb, lithium-oxide electrode yog qhov zoo electrode;rau titanate lithium-ion hlwb (LTO), lithium-oxide electrode yog qhov tsis zoo electrode.
Keeb kwm
Keeb kwm
Varta lithium-ion roj teeb, Tsev khaws puav pheej Autovision, Altlussheim, Lub teb chaws Yelemees
Cov roj teeb lithium tau thov los ntawm British chemist thiab tus neeg tau txais txiaj ntsig ntawm 2019 Nobel nqi zog rau chemistry M. Stanley Whittingham, tam sim no ntawm Binghamton University, thaum ua haujlwm rau Exxon hauv 1970s.Whittingham siv titanium (IV) sulfide thiab lithium hlau ua cov electrodes.Txawm li cas los xij, lub roj teeb lithium rechargeable no tsis tuaj yeem ua tau.Titanium disulfide yog ib qho kev xaiv tsis zoo, vim tias nws yuav tsum tau muab tso rau hauv cov txheej txheem kaw tag nrho, kuj tseem kim heev (~ $ 1,000 ib kilogram rau titanium disulfide raw khoom hauv xyoo 1970).Thaum raug huab cua, titanium disulfide reacts tsim hydrogen sulfide tebchaw, uas muaj ib tug tsis kaj siab tsw thiab lom rau feem ntau cov tsiaj.Rau qhov no, thiab lwm yam laj thawj, Exxon txiav tawm kev txhim kho ntawm Whittingham lithium-titanium disulfide roj teeb.[28]Cov roj teeb uas muaj hlau lithium electrodes qhia txog cov teeb meem kev nyab xeeb, vim lithium hlau reacts nrog dej, tso cov pa hluav taws xob hydrogen.Yog li ntawd, kev tshawb fawb tau txav mus los tsim cov roj teeb uas, es tsis txhob siv cov roj ntsha lithium, tsuas yog muaj cov lithium compounds, muaj peev xwm lees txais thiab tso tawm lithium ions.
Reversible intercalation hauv graphite thiab intercalation rau hauv cathodic oxides tau pom thaum xyoo 1974-76 los ntawm JO Besenhard ntawm TU Munich.Besenhard tau tshaj tawm nws daim ntawv thov hauv lithium hlwb.Electrolyte decomposition thiab hnyav co-intercalation rau hauv graphite yog qhov tsis zoo thaum ntxov rau roj teeb lub neej.
Kev loj hlob
1973 - Adam Heller tau npaj cov roj teeb lithium thionyl chloride, tseem siv rau hauv cov khoom siv kho mob cog thiab hauv kev tiv thaiv kab mob uas muaj ntau dua 20-xyoo txee lub neej, siab zog ceev, thiab / los yog kam rau siab rau huab cua kev khiav hauj lwm kub.
1977 - Samar Basu tau qhia txog electrochemical intercalation ntawm lithium hauv graphite ntawm University of Pennsylvania.Qhov no tau ua rau kev txhim kho ntawm lithium intercalated graphite electrode ua haujlwm ntawm Bell Labs (LiC6) los muab lwm txoj hauv kev rau lithium hlau electrode roj teeb.
1979 - Ua haujlwm nyob rau hauv ib pab pawg, Ned A. Godshall li al., thiab, tsis ntev tom qab ntawd, John B. Goodenough (Oxford University) thiab Koichi Mizushima (Tokyo University), tau qhia txog lub xov tooj ntawm tes lithium rechargeable nrog voltage hauv 4 V ntau yam siv lithium. cobalt dioxide (LiCoO2) raws li qhov zoo electrode thiab lithium hlau raws li qhov tsis zoo electrode.Qhov kev hloov tshiab no tau muab cov khoom siv hluav taws xob zoo uas ua rau cov roj teeb lithium ua lag luam thaum ntxov.LiCoO2 yog cov khoom siv hluav taws xob zoo ruaj khov uas ua haujlwm pub dawb ntawm lithium ions, uas txhais tau hais tias nws tuaj yeem siv nrog cov khoom siv tsis zoo electrode uas tsis yog lithium hlau.Los ntawm kev siv cov khoom ruaj khov thiab yooj yim-rau-kawg tsis zoo electrode cov ntaub ntawv, LiCoO2 enabled tshiab rechargeable roj teeb systems.Godshall et al.ntxiv txheeb xyuas tus nqi zoo sib xws ntawm ternary compound lithium-transition hlau-oxides xws li spinel LiMn2O4, Li2MnO3, LiMnO2, LiFeO2, LiFe5O8, thiab LiFe5O4 (thiab tom qab lithium-tooj liab-oxide thiab lithium-nickel-oxide cathode cov ntaub ntawv hauv 1985)
1980 - Rachid Yazami tau qhia txog qhov rov qab electrochemical intercalation ntawm lithium hauv graphite, thiab tsim cov lithium graphite electrode (anode).Cov organic electrolytes muaj nyob rau hauv lub sij hawm yuav decompose thaum them nrog ib tug graphite tsis zoo electrode.Yazami siv cov khoom siv electrolyte los ua kom pom tias lithium tuaj yeem hloov pauv hauv graphite los ntawm kev siv tshuab electrochemical.Raws li xyoo 2011, Yazami's graphite electrode yog cov khoom siv hluav taws xob ntau tshaj plaws hauv cov roj teeb lithium-ion lag luam.
Cov electrode tsis zoo muaj nws keeb kwm hauv PAS (polyacenic semiconductive material) nrhiav tau los ntawm Tokio Yamabe thiab tom qab ntawd los ntawm Shjzukuni Yata thaum ntxov 1980s.Cov noob ntawm cov thev naus laus zis no yog qhov kev tshawb pom ntawm cov khoom siv hluav taws xob los ntawm xibfwb Hideki Shirakawa thiab nws pab pawg, thiab nws tseem tuaj yeem pom tias tau pib los ntawm polyacetylene lithium ion roj teeb tsim los ntawm Alan MacDiarmid thiab Alan J. Heeger li al.
1982 – Godshall et al.tau txais US Patent 4,340,652 rau kev siv LiCoO2 ua cathodes hauv lithium roj teeb, raws li Godshall's Stanford University Ph.D.kev tshaj tawm thiab kev tshaj tawm xyoo 1979.
1983 - Michael M. Thackeray, Peter Bruce, William David, thiab John Goodenough tau tsim cov manganese spinel ua cov khoom lag luam muaj feem xyuam rau cov khoom siv cathode rau cov roj teeb lithium-ion.
1985 - Akira Yoshino tau sib sau ua qauv ntawm tes siv cov khoom siv carbonaceous rau hauv uas lithium ions tuaj yeem muab tso rau hauv ib qho electrode, thiab lithium cobalt oxide (LiCoO2) ua lwm yam.Qhov no ua rau muaj kev nyab xeeb zoo dua.LiCoO2 enabled industrial-scale ntau lawm thiab enabled lub lag luam lithium-ion roj teeb.
1989 - Arumugam Manthiram thiab John B. Goodenough nrhiav pom cov chav kawm polyanion ntawm cathodes.Lawv tau pom tias cov electrodes zoo uas muaj polyanions, xws li sulfates, tsim cov hluav taws xob ntau dua li cov oxides vim yog cov nyhuv inductive ntawm polyanion.Cov chav kawm polyanion no muaj cov ntaub ntawv xws li lithium hlau phosphate.
<mus txuas ntxiv…>
Lub sij hawm xa tuaj: Mar-17-2021





